
How can I oxidize #sf{Fe(OH)_2}# to #sf{Fe(OH)_3}#?
You can do so in basic #"pH"# by applying a positive voltage.
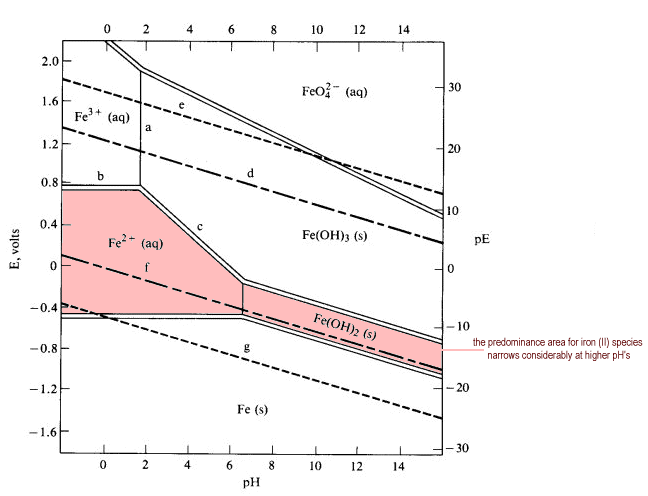
The above diagram is called a Pourbaix diagram, which plots voltage vs. pH. Higher pH is more basic, and higher voltage is more oxidizing (notice how the oxidation state of iron increases as we go upwards on the diagram).
To apply a positive voltage, you can use a second half-reaction in which the standard reduction potential #E_(red)^@# is positive enough that #E_(cell)^@ > 0.4# or so.
A choice that makes the overall reaction particularly spontaneous is to reduce hydrogen peroxide (since it is a good oxidizing agent, it can be easily reduced so that the iron complex is easily oxidized):
#"H"_2"O"_2(aq) -> "H"_2"O"(l)# (unbalanced), #E_(red)^@ = "1.78 V"#
#"Fe"("OH")_2(s) -> "Fe"("OH")_3(s)# (unbalanced)
Read further if you want to see how to balance and combine these half-reactions.
In general, to balance in base:
- Balance the oxygens with water molecules.
- Balance the hydrogens in water molecules using #"H"^(+)#.
- Add #"OH"^(-)# last to both sides and generate water.
- Cancel out the stoichiometrically-equal quantities of water on both sides.
- Balance the charge using electrons.
- Combine the half-reactions together.
To balance in acid, simply omit steps 3 and 4.
Reduction half-reaction:
#"H"_2"O"_2(aq) -> color(red)(2)"H"_2"O"(l)#
#"H"_2"O"_2(aq) + color(red)(2"H"^(+)(aq)) -> 2"H"_2"O"(l)#
#"H"_2"O"_2(aq) + cancel(2"H"^(+)(aq) + color(red)(2"OH"^(-)(aq))) -> cancel(2"H"_2"O"(l)) + color(red)(2"OH"^(-)(aq))#
#color(red)(2e^(-)) + "H"_2"O"_2(aq) -> 2"OH"^(-)(aq)#
Similar process (steps 1-5) for the oxidation half-reaction.
Oxidation half-reaction:
#color(red)("H"_2"O"(l)) + "Fe"("OH")_2(s) -> "Fe"("OH")_3(s)#
#"H"_2"O"(l) + "Fe"("OH")_2(s) -> "Fe"("OH")_3(s) + color(red)("H"^(+)(aq))#
#cancel("H"_2"O"(l)) + color(red)("OH"^(-)(aq)) + "Fe"("OH")_2(s) -> "Fe"("OH")_3(s) + cancel("H"^(+)(aq) + color(red)("OH"^(-)(aq)))#
#"OH"^(-)(aq) + "Fe"("OH")_2(s) -> "Fe"("OH")_3(s) + color(red)(e^(-))#
Combined reaction:
#cancel(2e^(-)) + "H"_2"O"_2(aq) -> cancel(2"OH"^(-)(aq))# #2(cancel("OH"^(-)(aq)) + "Fe"("OH")_2(s) -> "Fe"("OH")_3(s) + cancel(e^(-)))# #"-"#
#color(blue)("H"_2"O"_2(aq) + 2"Fe"("OH")_2(s) -> 2"Fe"("OH")_3(s))#
Remember to make sure the electrons cancel out.
Link nội dung: https://truyenhay.edu.vn/feoh2-feoh3-a59371.html